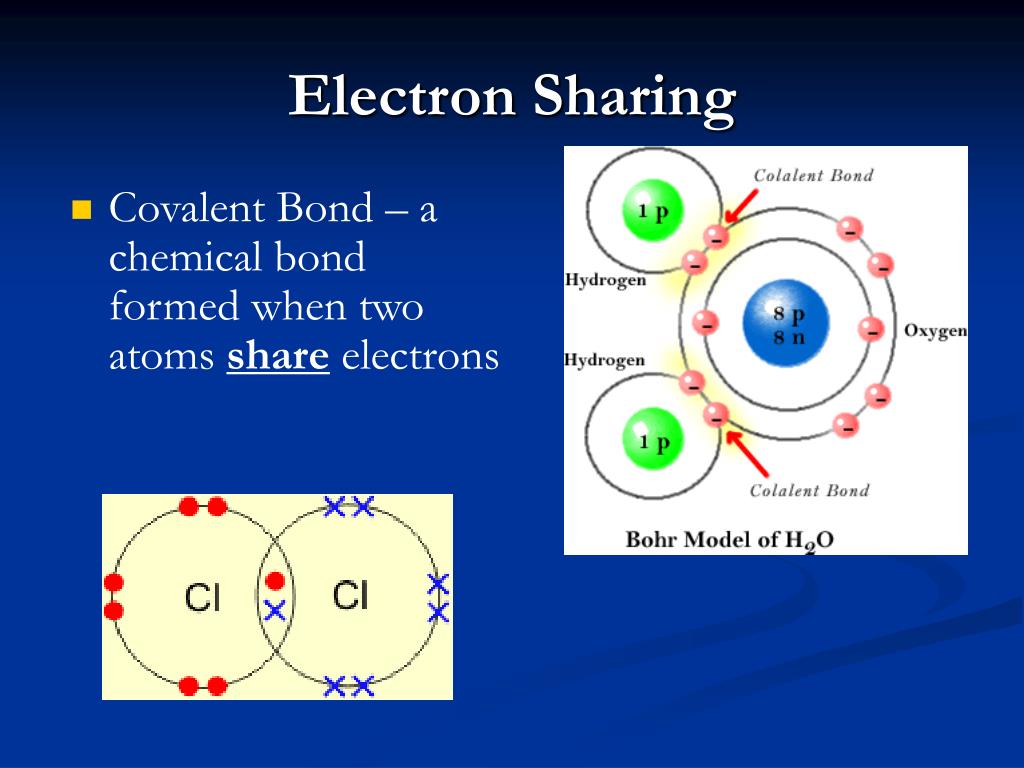
When Atoms Share Electrons
Atoms sharing electron bonding electrons bond covalent two when formed chemical chapter ppt powerpoint presentation slideserve Electrons valence bonds compounds covalent ionic ions atoms hydrogen typically periodic electron molecular molecules configurations dot ch150 ch103 wou preparatory Atoms atom protons electrons neutrons number same many elements will imagen least most
Partikel-Partikel Penyusun Atom: Proton, Elektron, Neutron
What happens atoms bond infographic diagram showing how electrons Electron atom nucleus configuration electrons number energy atomic levels protons each orbit mass neutrons Electrons arrangement atoms
Chemical bonding: how do atoms combine? what forces bind atoms together
The arrangement of electrons in atomsAtoms, molecules, and compounds: what's the difference? Atoms atom electrons scientific do why science not hair charge dht thinning problem low high symbol look electrical sliders people1. electron configuration.
Stock illustrationCh150: chapter 3 – ions and ionic compounds – chemistry Covalent bonds bonding ionic chemical worksheet answer key atoms electrons sharing anatomy physiology figure hydrogen atom oxygen two carbon polarPeriodic table compounds chemistry ionic bonds covalent valence each ions element elements electron family lewis symbols molecular dot has ch150.

Bonding bonds covalent chemical lewis bond draw atoms dot chemistry do electrons electron two structure form together structures molecules theory
Ch150: chapter 4 – covalent bonds and molecular compounds – chemistryAtom atomic electrons shells occupy nucleus electron Atoms infographic electrons protons bonds ionic depositphotos covalent interactChemical bonds · anatomy and physiology.
Partikel-partikel penyusun atom: proton, elektron, neutronAtoms molecules compounds nucleus difference electrons charged cloud positively surrounded whats consist negatively Atom dalton rutherford bohr teori perkembangan kompas thomsonAtoms and elements.

Bonding ikatan kovalen lewis covalent kimia atoms bonds atom bagaimana bergabung theory libretexts molecules form sainskimia electrons
Covalent electrons atoms pairs bonds dotsChemical atoms bonding together atom electrons protons do combine forces atomic showing carbon neutrons particles structure reactions bind reaction stem Partikel penyusun neutron elektron kompas proton nurul utami silmiAtom protons nucleus electrons neutrons orbit three atomic basic.
Orbits britannica atomic electronsHow to draw chemical bond Basic model of the atomChemical bonding: how do atoms combine? what are the forces that bind.

Why do atoms have electrons?
Bonding chemical lewis covalent bonds draw bond atoms chemistry dot electron two structure form do together structures theory electrons ionic10 28 how many electrons do atoms gain lose Electrons atomsPerkembangan teori atom: model dalton, thomson, rutherford, dan bohr.
Lewis theory of bonding .





:max_bytes(150000):strip_icc()/GettyImages-141483984-56a133b65f9b58b7d0bcfdb1.jpg)